In the final installment of my “Let’s Make a Drug” series, I’d like to talk about another exciting and complex topic in modern drug making: Delivery.
But first if you’re thinking to yourself, “isn’t FDA regulations and clinical trial design more important? Why is delivery of all things the third topic?” That’s a great point. And my answer to that is simply:
- I don’t know enough about it to speak on it.
- Even if I did… as a Scientist, I think the science is way more interesting.
That’s for the Chief Medical Officer to know, and why they’re gonna get paid the big bucks.
Today, we’ll talk about the real headaches of drug making – once you find your drug (target) and a great model to test it in, how do you put it in the specific organ or cell that you want?
We’ll start by first setting the context of why delivery matters in the first place, briefly going over traditional drug classes (that include your Aspirin, Lexapro, statins, etc.) and their delivery methods. We’ll then discuss the new generation of drug classes and their unique challenges around delivery. Finally, we’ll bring it all back together to talk more holistically about the Drug Making process, ending with what’s in store for the future. This one’s going to be a bit shorter so I can spend a little extra time bring it together at the end.
And if you’ve been reading along the past three weeks… Thank you, thank you, thank you. Your support means the world to me and hopefully you’ve been seeing some improvements in my writing And if you’re new here, Welcome!!! I hope you enjoy what you read today.
Okay… Let’s get into it!
Harder, Better, Faster, Stronger:

In this century, we’ve seen a revolution in the types of drugs we can create – and we’re just getting started. The Drug Hunters dived head-first into the rabbit hole of biology and found incredible systems to leverage into miracle technologies.
Gene editing, DNA sequencing, directed evolution… these enormous leaps in science have allowed us create classes of drugs (modalities) that are ancestors would’ve only dreamed of. These modalities like gene and cell therapies are becoming better, faster and stronger than anything we’ve seen before. But they’re also bigger, more complex, and potentially deadly.
But how did we get here? It all started with drugs like Aspirin.
Aspirin was one of the first pharmaceutical drugs ever made. A class of drugs that we now call “small molecules,” their low molecular weight (<1000 Daltons) and relatively simple construction (20-100x atoms) makes them incredibly compact and stable at room temperatures.
These small molecules are so, well, small, that they are often delivered into cells by a process called “passive diffusion.” This is when molecules move freely in and out of cells from an area of high concentration to low concentration, just like osmosis!
Small molecules are great. They’re room temperature stable, no needles, and you don’t have to worry about sterility either. I’ve definitely taken some loose Advil in my backpack once in a real pinch, and I lived to tell the tale. But with all drugs, there are drawbacks. And with small molecules, one of the major problems are off-target effects.
We’ve all heard it before – the drowsiness after the Benadryl, ulcers from Advil, muscle pains from statins… some of them are whatever, some are scary, and some just don’t make any sense. It’s a serious issue, and many of these molecules usually impact the GI track because they’re usually first digested from the stomach.
Side effects become increasingly dangerous as the pathways you target become more critical to regular bodily function. Doxorubicin for example is a common anti-cancer drug that fights cancer by stopping growth of cells. But here’s the catch: it’s not specific to which cells it stops… That’s why you lose hair on chemotherapy drugs and why many small molecules can be harmful – they’re not specific enough.
The crux of modern drug making is therefore not how effectively your drugs fight the disease, but how accurately you can do it.
Delivering Drugs to The New Frontier
In this New Frontier of drug discovery, we’ve started to create medicines that redefine the concept of a “drug.” We’ve been changing cell pathways and interactions at the molecular level for two centuries. We’re now moving onto DNA medicines that can fix our bodies at the most fundamental, genetic level.
The DNA is The Cure, and we’ve created it in our own image to fight disease. Zolgensma, a gene therapy, allows the insertion of the survival motor neuron 1 (SMN1) gene to give children with Spinal Muscular Atrophy a chance at a normal life. Luxturna, a gene therapy for the eye, restores partial vision to blind patients with a genetic mutation in gene RPE65.
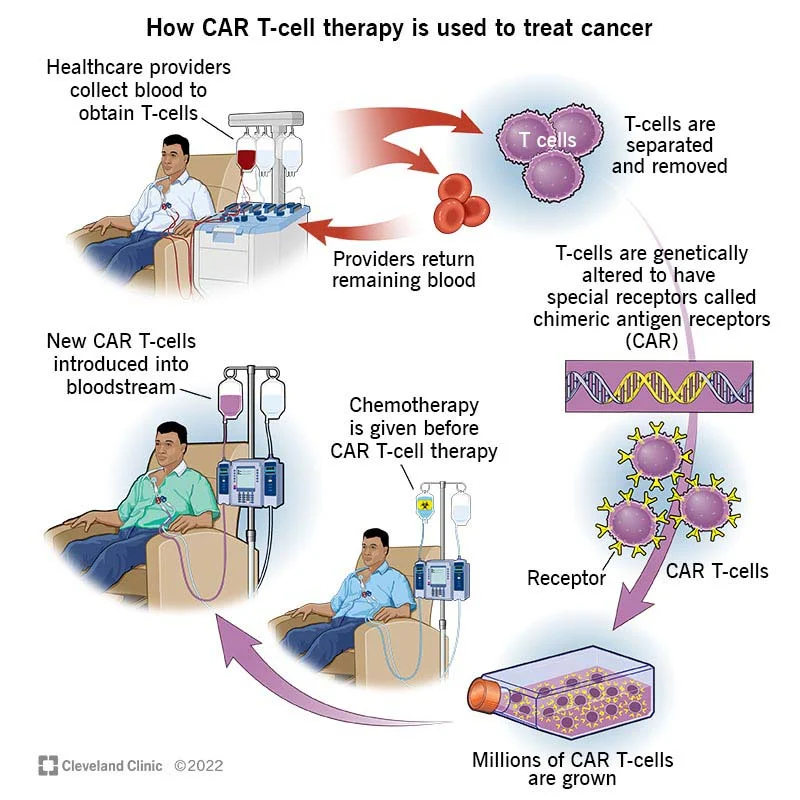
This technology has even given us new ways to fight cancer. We can now reprogram our own immune cells to fight cancer. Called Chimeric Antigen Receptor – T cells (CAR-T), this chemo-free cancer therapy allows patients to use their own immune cells, previously unable to recognize their cancer, and reprogram them to fight cancer once again. These treatments are truly effective and, most importantly, safe. Just recently we witnessed one of the first girls who received this cancer therapy a decade ago, enroll into college – University of Pennsylvania. The same institution that, coincidentally, cured her from cancer.
Every year we’re seeing more therapies of this kind in the clinical pipeline. 58 gene and 21 cell therapy trials have been initiated in this past quarter alone, with thousands more to be submitted by 2030.
Gene and cell therapies are the future. With this technology, Scientists are now going after some of the greatest diseases of our kind. Using large(r), complex payloads like plasmid DNA, mRNA, and proteins, they allow us to target specific genes, inside specific cells, inside specific organs, exactly how we want.
We’re now going after complex diseases – conditions from Osteoarthritis to pain and aging – that we previously thought were untreatable. In this decade, we’re going to start seeing dozens of these therapies targeting entire organ systems in clinical trials.
But even though we’ll see huge technological progress in gene therapy payloads along the way, the delivery cargos must become even more sophisticated.
Delivering gene and cell therapies:
In the early days of aviation, there were lots of different shapes and sizes of airplanes. But now when you look on the tarmac, all the planes look basically the same. As with many inventions in history, the search for maximum efficiency forces things to adopt specific hyper-optimized shapes and sizes.
The field of genetic medicine is the same. As of today, the field is dominated by a specific delivery system: viral vectors. Specifically, Adeno-Associated Viruses (AAV).
Going viral
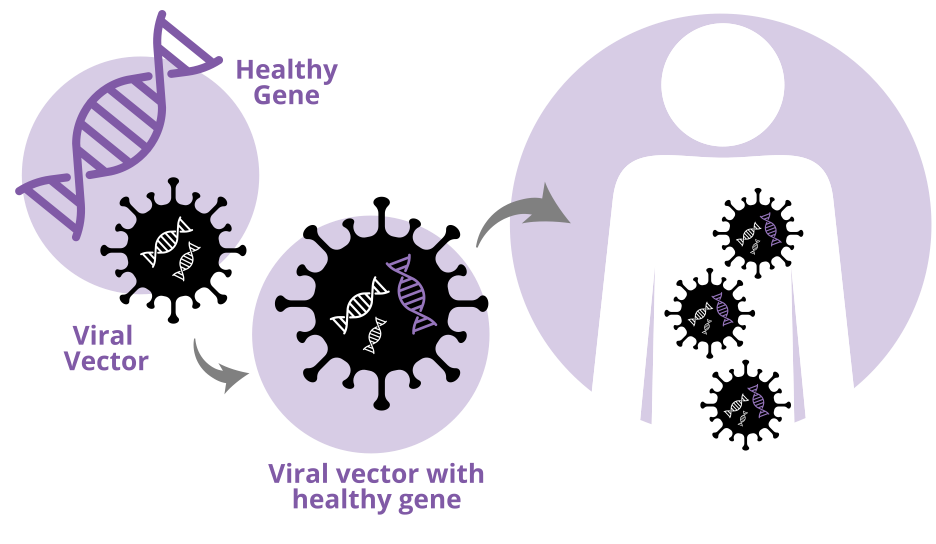
Most Gene Therapies use a type of virus called adenoviruses that is emptied out of its gross and harmful viral components and repackaged with our drug payload. It’s like taking a creeper van, ripping outthe guts, and doing a complete glamping remodeling to enable your dream of being a digital nomad. Scientists do exactly that, except with drugs!
Like airplanes, these AAV vectors come in relatively fixed shapes and sizes. They have a 20-sided (icosahedral) shell, with fixed size limits (~5,000 DNA bases), and need a rep, cap, and aap gene to function. Of course, you can still modify them, and that’s why AAVs are so attractive. These vector shells can be modded out in different ways to make them organ and even cell type specific. AAV9 for example work well in the CNS, and clinical trials have started with AAVrh10 in patients with Frederick’s Ataxia in heart. AAV8 is great for liver, and AAV6.2 is being used for to try to treat Cystic Fibrosis in the lungs.
The simple, effective, yet customizable nature of the virus has made it the delivery vehicle of choice for gene and cell therapy specialists around the world, but there are still many drawbacks. Namely, once again, off-target effects.
These drugs, also called precision medicines, are often injected directly into your bloodstream and land at the specific diseased cells/organs. But naturally, they pass through your entire body and inevitably stick to some organs along the way. For AAV gene therapies at super high concentrations, these acute toxicity events are one of the major unsolved problems in the field.
Liver toxicity, immune responses, and rare integration events are the main “side effects” that we have to consider during AAV gene therapy administration. These aren’t actually “side effects” per se, because clinicians often extensively pre-screen patients to make sure they don’t have any pre-existing liver or immune conditions (often these are exclusion criteria). However, while these reactions are incredibly rare, they are often fatal when they happen.
As of today, there still doesn’t seem to be a great way to avoid AAV toxicity altogether, especially for the liver. CpG deletion, TLR9 inhibition, capsid mutation, immunosuppressant co-injections; there’s been several methods that all work to reduce these effects to some extent, but still does not eliminate it.
Entire companies now exist solely to find extremely effective vectors for specific cell types or organs. Even more companies and academic labs are creating non-viral delivery methods that allow for even larger payloads and more specific delivery to organs. If we’re going to stick with viral vectors to fight diseases like Heart Failure and Schizophrenia, we’ll need to find better solutions to AAV off-target effects along the way.
The Druggable Target is not The Drug
In this century, we’ve been given new tools to try to truly cure disease. We’re past the proof-of-concept stage. Way past it, actually – we’ve already succeeded in treating monogenic diseases like Hemophilia A/B with gene therapies. By 2040, we’re going to have treatments for several dozens, maybe +100 monogenic diseases that we thought were untreatable in the previous century.
But what’s after that? Non-monogenic, complex diseases.
Curing diseases like HFpEF, OA, diabetes and cancer, will be some of the greatest achievements in medical history. But it’s not going to be easy. To do this right, we’re going to need use every single tool in our reach, rally up the brightest minds of our time, and put a lot, of money into it.
We’ve also learned a lot of lessons along the way, often times the hard way.
But as we head towards the New Frontier to cure our greatest maladies, there’s one lesson stands out: The Druggable Target is not The Drug.
Perturb-seq, DNA editing, AI, are powerful. But they’re just tools at the end. They might give you a new gene or a pathway you can change to restore healthy function to a patient, but that target is not your drug.
You’ve found your target using pooled screening. Okay, but how exactly will you perturb it? Plasmid DNA? mRNA? Recombinant antibodies? CRISPR/Cas9-RNPs?
What about the delivery vehicle? You can go viral, but which one? Within AAV there’s AAV2, 4, 6, 8, 9, rh10, MYO, etc. But there’s also lentiviruses and even bocaviruses too. Don’t forget non-viral methods like lipid nanoparticles, gold nanoparticles, exosomes, hydrogels, or just straight-up DMSO?
And where are you going to test that? I’m pretty sure I’ll get Heart Failure in the future, but if me and my friends are getting a gene therapy for it, I really hope it was developed in a great animal model.
In this New Frontier of drug making, every single piece has to be perfect. Finding the target is only the beginning, and it’s up to us to define the path forward. The right delivery method, vmodel, clinical trial, all must come together to make this work.
But no matter the obstacles, we’re going to succeed. With the right science and the right team, we will cure the most horrible maladies of our kind. By the end of this decade, we’re already going to see some of the first drugs to treat these incredibly complex diseases.
I’m bullish, and I hope you are now too.
Afterword: Moving (kind of) to Substack
Thank you everyone for joining me in my blog series. Thank you to everyone for your support, encouragement, and feedback along the way. With the end of this series, I want to officially launch my new, more focused, endeavor – The New Frontier.
This is a Substack page that I will be writing slightly longer pieces and series like these. It’s essentially a duplicate except the UI is much better and it’s easier to track reader engagement and stuff. Ultimately, I think platforms like Substack would be a better way for me to understand my audience as I (hopefully) scale.
If you enjoy what you’ve been reading but don’t want me to remind you when I post every time… just Subscribe to my Substack! 🙂 That way whenever I post, a notification will automatically go to your email.
Thank you everyone for your support, and we’ll talk again next week! Same time, same place.