Models, Models, Models
Curing a disease starts and ends with models.
Animal models allow Scientists to replicate a disease and study it in a living organism in all of its complexity. These animals are the unsung heroes of the Drug Hunter story, and their purpose, use cases, and contributions are misunderstood. In Part 2 of my “Let’s Make a Drug” series, today, I want to talk about the most exciting and nuanced part of drug discovery: Animal models.
Intro
Why haven’t we cured cancer? Diabetes? Dementia or Heart Failure?
Trillions of dollars spent over decades of research, and we still live in a world riddled with these maladies. But why is that? The Scientists aren’t slacking off, and “the government” isn’t keeping any secrets from you either. The simple truth is… if we knew how to cure a disease, we would’ve cured it by now.
No one’s keeping secrets, and Scientists want the answers just as bad as you do. But the harsh reality is that the next breakthrough drug for complex disease starts and ends with a great animal model.
But let’s backtrack and talk about why we need to use our furry friends for drug discovery in the first place. How did we get here? How hard is it really to find a great model? Why don’t we just use AI? We’ll answer all of that and more. Let’s get into it!
From now on, I will refer to animal models as simply “models”
Animals <> Drug Discovery
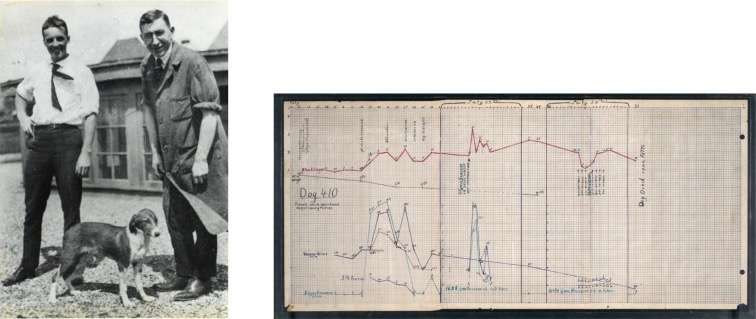
Cures to most complex diseases are found through great models. The discovery of insulin in 1921 for example would not have been possible without dogs. While primitive, Best & Banting realized that injecting pancreas extract into dogs without a pancreas could lower their blood sugar and prolong their life. These were the first successful in vivo (inside living animal) experiments that led to the commercial production of insulin for diabetics just two years later in 1923.
Similarly, polio could not have been cured without the help of Rhesus monkeys either. They were exceptional at modelling the immune and nervous system effects of the virus, and without them we wouldn’t have been able to find a vaccine as quickly as we did. As of today, over 18 million people have been saved from paralysis or death because of these monkeys. Without their help, many of us might’ve lived our lives in an Iron Lung.
We need animal models because human diseases are too complex to simulate/replicate anywhere else. Diseases and its symptoms usually arise from some combination of your immune system, metabolism, central nervous system, genetics, and a whole lot more. They’re often too complex to stimulate in a petri dish (in vitro) or on a computer (in silico), and Scientists can’t experiment on diseased or dead patients (or at least not anymore). What’s worse, lots drugs that look like they do something in vito/silico don’t do translate in vivo, a mistake that could cost billions of dollars.
That leaves animal models to be the best and usually only way to study the disease in its natural environment without compromising the science, human safety, or FDA regulations*.
It’s a tried-and-true approach. In the past ~100yrs, animal models have directly and indirectly given us antibiotics, vaccines, and cures to many metabolic and simple genetic diseases. And over the next several decades, we will continue to rely on them to relieve us from the greatest maladies of our time. But as we’ve started fighting increasingly complex diseases, we’ve started to hit a wall… We can’t find great models anymore.
No Role Modelz (for Complex Disease)
Of the ways to discover a drug, tinkering around mapping out individual cell pathways no longer lead to drug discoveries in this century. Most of the simpler diseases have already been cured or are on their way to be within this decade. But the more complex diseases like cancer or dementia are a different story.
Diseases are the mistakes of biology. They’re the bugs, or rare errors that accumulate overtime through millions of correct repetitions of cell signaling. It can be irreversible DNA repair from UV radiation, the tasty carcinogens in those Cowboy Killers, or that third In-N-Out 3×3 of the week with extra toasted buns, Animal Style.
It’s hard to say where these complex diseases actually come from. Heart Failure for example is strongly correlated with Diabetes, where despite it being a heart condition, it almost looks like a metabolic disease. Dementia on the other hand is still a black box. Alzheimer’s is the most common type of dementia, but even for that there’s still only strong hypotheses, no concrete mechanisms, for why it happens in the first place.
In the modern hunt for curing complex disease, a great model is the single biggest missing piece to this enormous puzzle.
If we had a great model, we could start dissecting the complete molecular circuit to find incorrect pathways that cause disease. We could start to understand what specifically went wrong first, and stop it before the real damage happens. We just need one great model, and we have a shot at curing the whole disease. It all starts and ends with one great model.
Finding the next top model

No price is too high for a great model. In modern drug discovery, an exceptional animal model can make or break a drug’s success in a clinical trial. In Heart Failure (with preserved Ejection Fraction) for example, we have many good models but no great ones. Dozens of physicians and scientists are looking for new HFpEF models, but they each have distinct flaws.
So when you’re testing your drug in preparation for a human clinical trials, you better choose wisely. There are largely two ways to think about animal models:
- How does the disease progress?
For complex diseases like HFpEF, we still don’t know exactly how it develops in humans, but it doesn’t naturally occur in animals either. So, the only option we have is to induce it ourselves. But how can we recreate a disease in an animal when we don’t completely understand it yet?
Well, we do it by breaking it down into individual things wrong we know about the disease. HFpEF patients for example have high blood pressure, so we induce it with hypertensive drugs like L-NAME or DOCA. Hypertrophy? Aortic banding. Many HFpEF patients have obesity/diabetes? Give the animal a high fat or Western diet.
Scientists use this sort of “multi-hit” approach where the model receives damage to individual body systems that then re-create the complex disease from the sum of its individual injuries. The thought is that although the way the damage occurred was not natural like in humans, the individually damaged body systems come together such that the global physiology of the disease mimics that of a human.
But the real concern is when damage is introduced in an artificial way like this: is it still the same disease?
This brings us to the second point of translation.
2. Will the drug translate between models?
If HFpEF is introduced in such an artificial way in a Göttingen pig, is it really going to be comparable enough to the human version? We don’t know how our version of HFpEF develops, so will giving a pig DOCA really reproduce our version of the disease? HFpEF is also more common in women. But if we can only model it in male mice, is that really a good model?
The real danger of a “good” model is therefore the likelihood of it giving you a false positive result, as in, it works in the animal but not in humans. You don’t want your drug to fix the specific mechanical damage your model received; you want it to cure the actual disease itself.
Similarly, false negative results can be just as bad. What if your drug doesn’t do anything in a mouse, but works in a pig? Or worse, what if it doesn’t work in either mouse or pig, but actually works in humans?
False negative results can make us shelve the drug and throw it away in the library of “failed drugs.” After several decades of drug hunting, it’s impossible to say how many drugs have been tried and scrapped because they looked like they “didn’t work” in one animal, when it actually could’ve worked in the other.
Drug making is a high-stakes game, and mistakes like these is out of the question. A drug failing in Phase II/III clinical trials could mean >$1 billion in investment lost and your company spiraling down the abyss – a mind-boggling but all-too-common occurrence in modern drug making. In the search for curing complex diseases, creating and selecting animal models is one of the most difficult decisions you’ll make. If you ask me, I’d say go natural whenever possible:
Go natural

Natural is King
Given the complexity of artificially induced disease models, if you can, going natural makes a lot of sense. Naturally occurring disease models are often the best ones because it captures the variety and nuance we see in patients with complex disease. Racing horses and camels for example naturally get osteoarthritis in their lifetime, making them an excellent natural model. Cats and dogs also live a very similar life to their human companions and are exposed to many of the same air-borne toxins and chemicals, making them (sadly) better models of naturally occurring idiopathic lung fibrosis.
Why bother with models at all?
With Large Language Models and AI being at the forefront of the news lately, it’s only natural to ask: why can’t we replace animals’ lives with computer simulations?
An “AI algorithm” can only output the quality data that it was trained on. If the Schizophrenia transcriptomic data we give the AI for example is wrong, it’s going to give us wrong answers – false positives. It’s also unclear how we can relate the pharmacokinetics of a drug between å mouse model and human completely in silico.
This is not the decade of end-to-end in silico drug discovery. Computers are absolutely essential in helping us untangle complex high-dimensional data, but they cannot replace animals as drug testing environments for another three decades at least. Our human understanding of biology and disease are simply not there yet.
But leaps in technology like pooled screening and federal policy (like the FDA Modernization Act 2.0) have started to chisel away at the ~two-decade gap between the time of drug discovery and commercialization. Even if we’re still a few decades away from end-to-end AI/ML drug discovery, other technology is evolving super quickly.
3D culturing of diseased human tissues, Organ-on-a-Chip, heart-in-a-box, cryopreservation, innovation is happening at every corner to supplement and/or replace our furry friends. By the end of this century, we all dream of a future where we no longer need the help of animals to cure our diseases.
Final thoughts:
It doesn’t matter if you know how to look for drugs, if you don’t have a great model to test it in. At best, you’ll fail at finding anything that works. And at worst, you’ll run a clinical trial with the drug, spend $2B, then fail.
In this New Frontier of drug making, every single decision counts, especially model selection. And of course, it should. If millions of people’s lives are on the line, the data better be real.
So next time you read a sensational headline saying “New Drug by (insert big phrama name) Might Stop Aging!?” dig a little deeper to see what model they tested that drug in. Maybe it was on a petri dish, or maybe it was all in silico… in which case, I’d take it with a big fat grain of salt.
But what do you think? Do you think we’re ready to move towards end-to-end drug development with AI? Could we make do with the models we already have? Let me know in the comments!
(Check out dat new comment feature!! Totally anonymous and hopefully decent WordPress UI)

